ICARIA, Sierra Leone
ICARIA = Improving Care through Azithromycin Research for Infants in Africa

Overview
An evaluation of the impact on childhood mortality of azithromycin plus perennial malaria chemoprevention administered through the Expanded Program of Immunisation in Sierra Leone.
Objectives
General objective
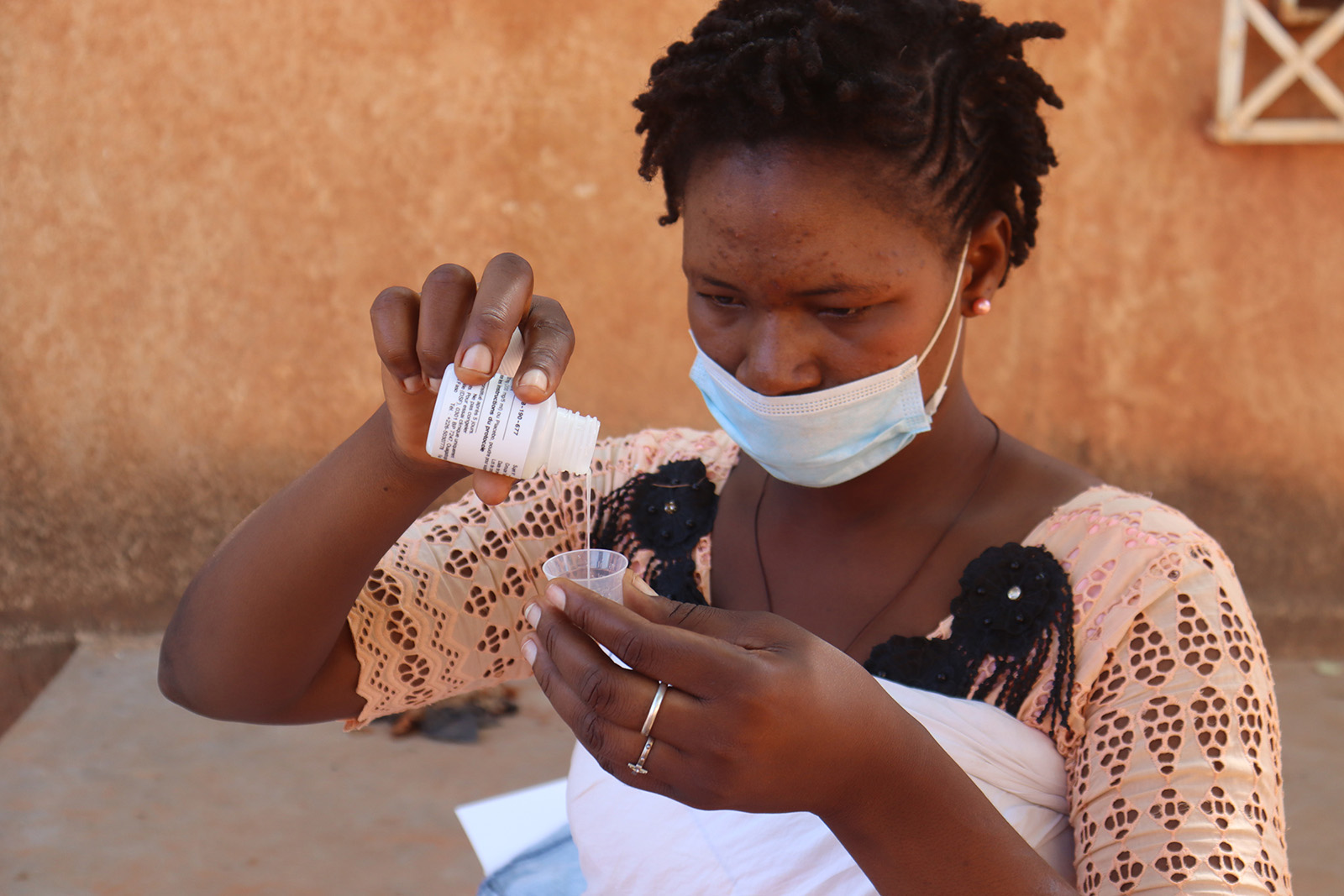
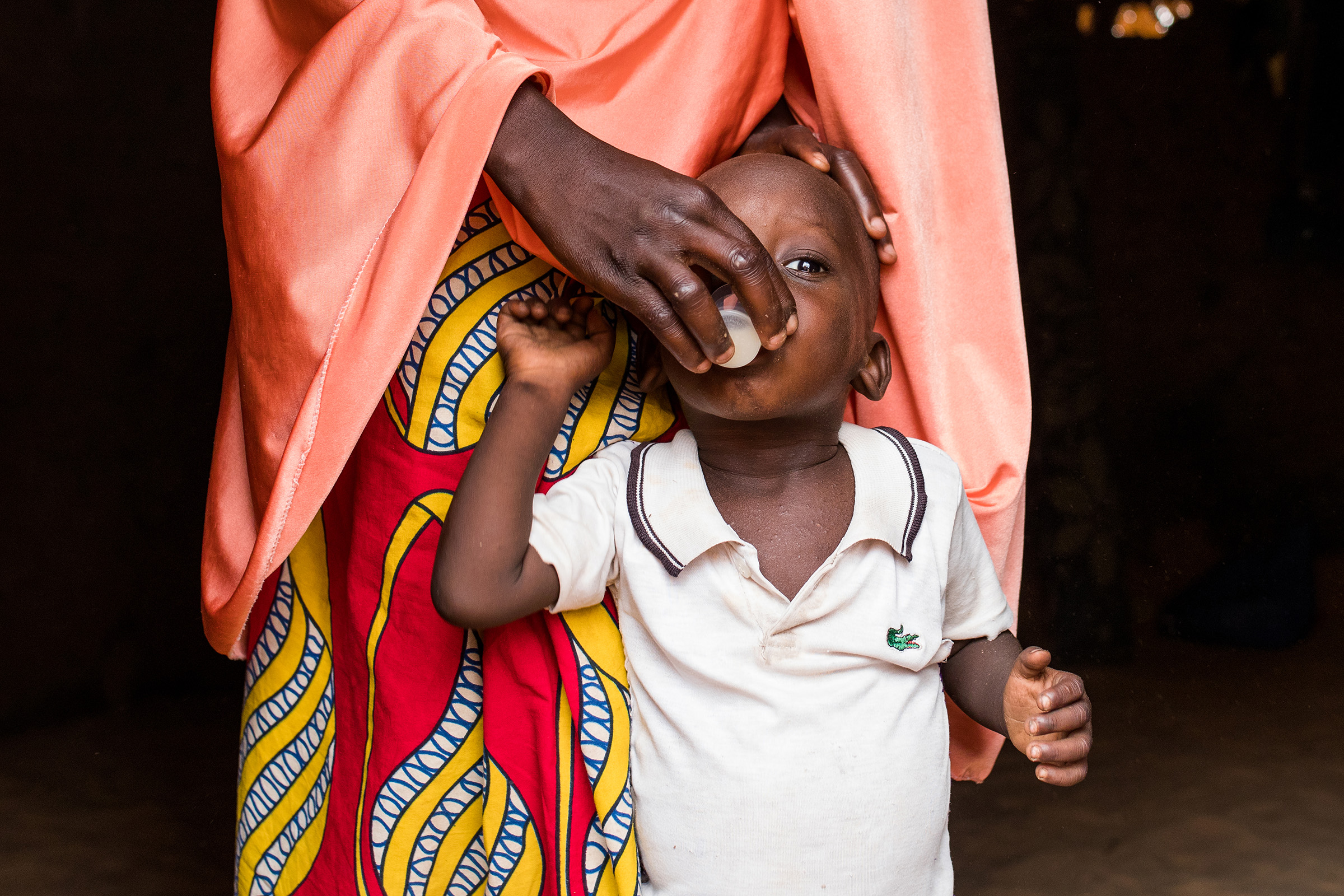
To evaluate the impact of azithromycin administered through the Expanded Program of Immunization in addition to perennial malaria chemoprevention (PMC) with sulfadoxine pyrimethamine (SP) on all-cause mortality at 18 months of age in Sierra Leone.
Specific objectives
1.
To evaluate the effect on all-cause mortality up to the age of 18 months of adding azithromycin at 6 weeks, 9 and 15 months of age to PMC administered at 10, 14 weeks, 6, 9, 12 and 15 months of age, alongside routine EPI immunizations.
2.
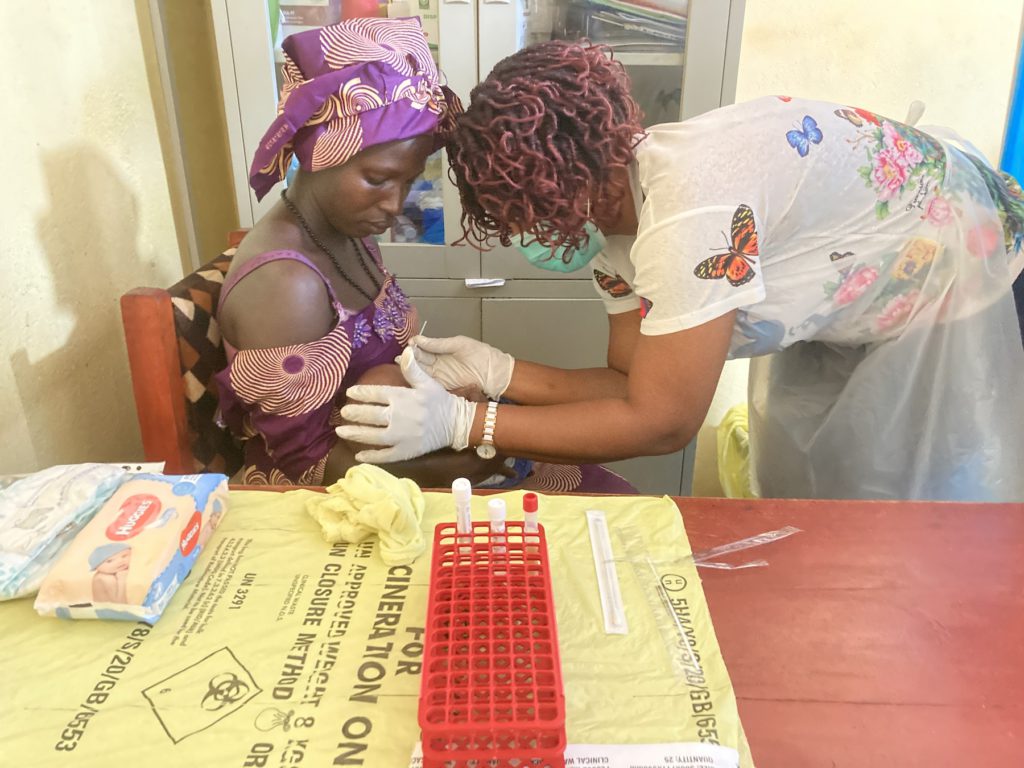
To assess adverse events associated with azithromycin administration.
3.
To assess the potential development of resistance to macrolides.
4.
To determine the potential interactions between azithromycin and specific routine EPI immunizations (Measles, Rubella and Yellow Fever).
5.
To determine the effect of the interventions on malaria-related indicators.
6.
To assess the potential risk of development of SP parasite resistance.
7.
To examine the health system implications related to the addition of azithromycin to the EPI and its acceptability to health providers and participants.
Study design
A Phase III individually-randomized, double blind, two- arm placebo-controlled trial of azithromycin/placebo administration in young children in Sierra Leone.
Study location
The trial is being conducted in three districts of Sierra Leone, namely Bombali, Port Loko and Tonkolili, and 14 primary health care facilities.


Sample size
A sample size of 20,560 infants (10,280 per group azithromycin / placebo) has been estimated to achieve a 16.6% reduction in all-cause mortality at 18 months of age, with 80% statistical power and an alpha error of 0.05.
Primary study endpoint
All-cause mortality rate at 18 months of age.
Key milestones
- First child recruited on 17 March 2021
- Last child recruited on 25 April 2024
- “End of follow-up” completion as of 9 October 2024: 13,205 children
- “Active follow-up” of children as of 9 October 2024: 6,534 children
- Expected “end of follow-up” completion in October 2025.
ICARIA Key Personnel & Partners
Study International Principal Investigator
Prof. Clara Menendez
Study Local Principal Investigator
Prof. Mohamed Samai
Study partners (institutions):
Barcelona Institute for Global Health (ISGlobal)
/ Hospital Clínic – Universitat de Barcelona, Spain
Ministry of Health,
Government of Sierra Leone
College of Medicine and Allied Health Sciences (COMAHS),
University of Sierra Leone