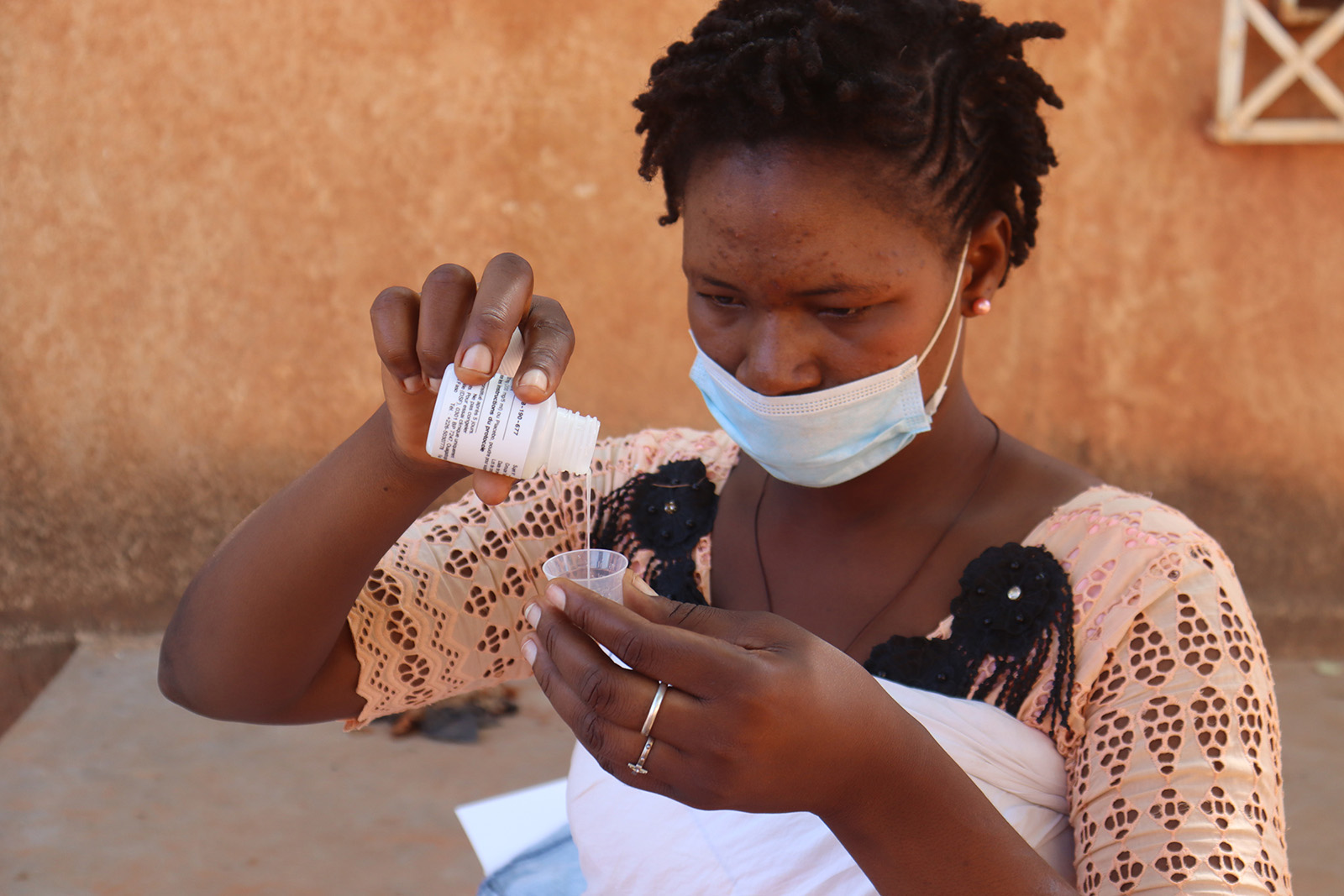
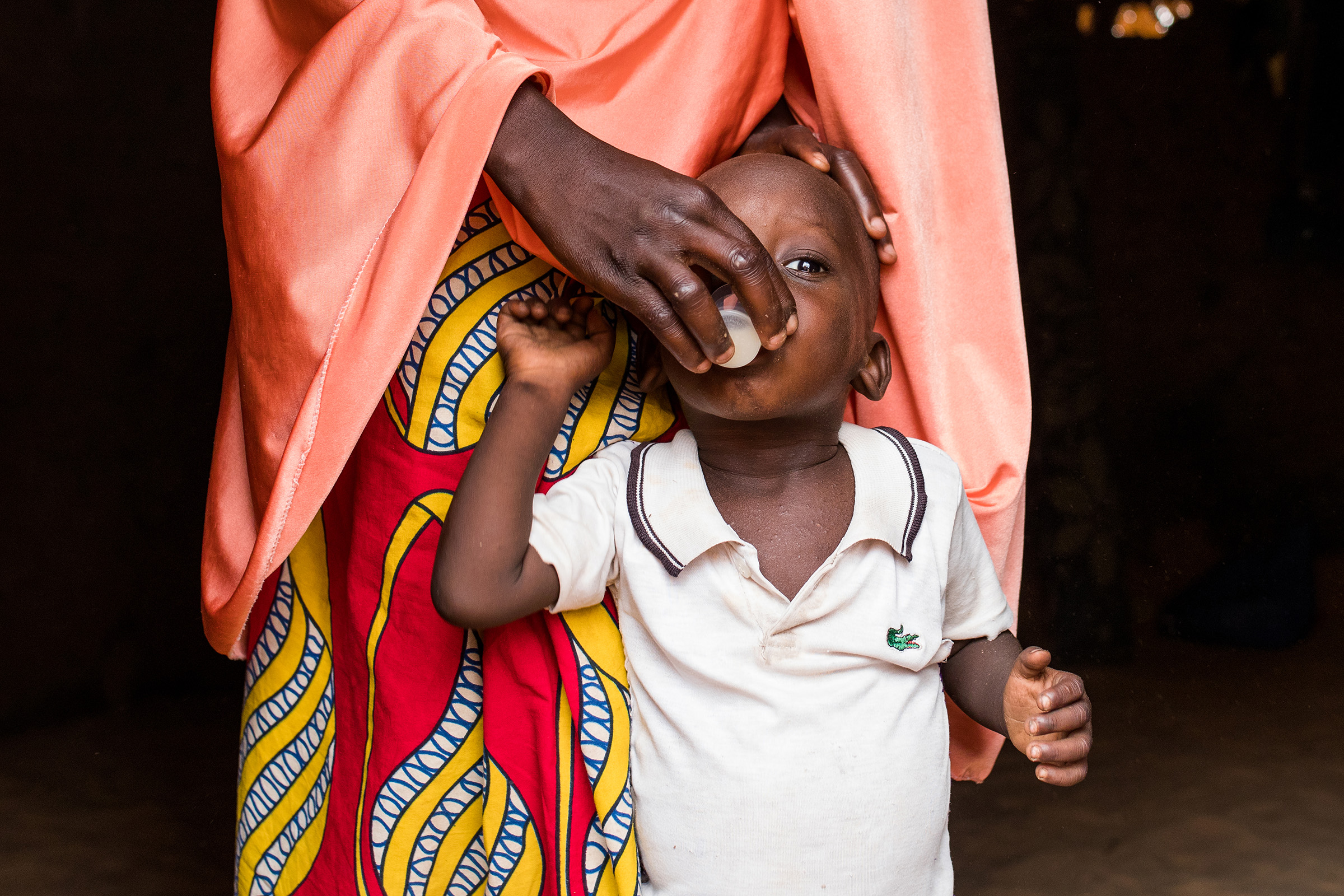
SARMAAN, Nigeria
SARMAAN = Safety and antimicrobial resistance of mass administration of azithromycin among children aged 1–11 months in Nigeria

SARMAAN I
SARMAAN I is a hybrid Implementation Science project aimed at improving child survival and helping to fast-track the attainment of Sustainable Development Goal (SDG) 3 in Nigeria.
The project entails the mass drug administration of Azithromycin to all children aged 1–11 months in communities with high infant mortality, per WHO guidelines, alongside continuous monitoring of safety and antimicrobial resistance patterns in Nigeria.
The project generates vital information on the cost, cost- effectiveness, feasibility, and acceptability of using different platforms: Neglected Tropical Disease-Trachoma (NTD- Trachoma), National Program of Immunization (NPI), the Polio Elimination Initiative (PEI) and the Seasonal Malaria Chemoprophylaxis (SMC) platforms, to guide scale-up and policy in Nigeria.
The Nigerian Institute of Medical Research leads the research work in collaboration with implementing partners, state governments (Kebbi, Sokoto, Jigawa, Kano, Akwa Ibom and Abia) and allied agencies of the Federal Ministry of Health and Social Welfare for prompt translation of findings into policy.
Objectives
- To determine the effect of mass administration of azithromycin among children 1 to 11 months old on the safety and pattern of antimicrobial resistance in all project sites.
- To determine the cost, cost-effectiveness, feasibility and acceptability of delivering azithromycin across platforms.
Study design
- Repeated cross-sectional for AMR (Research)
- Biannual distribution of MDA AZM in all sites except Kano.
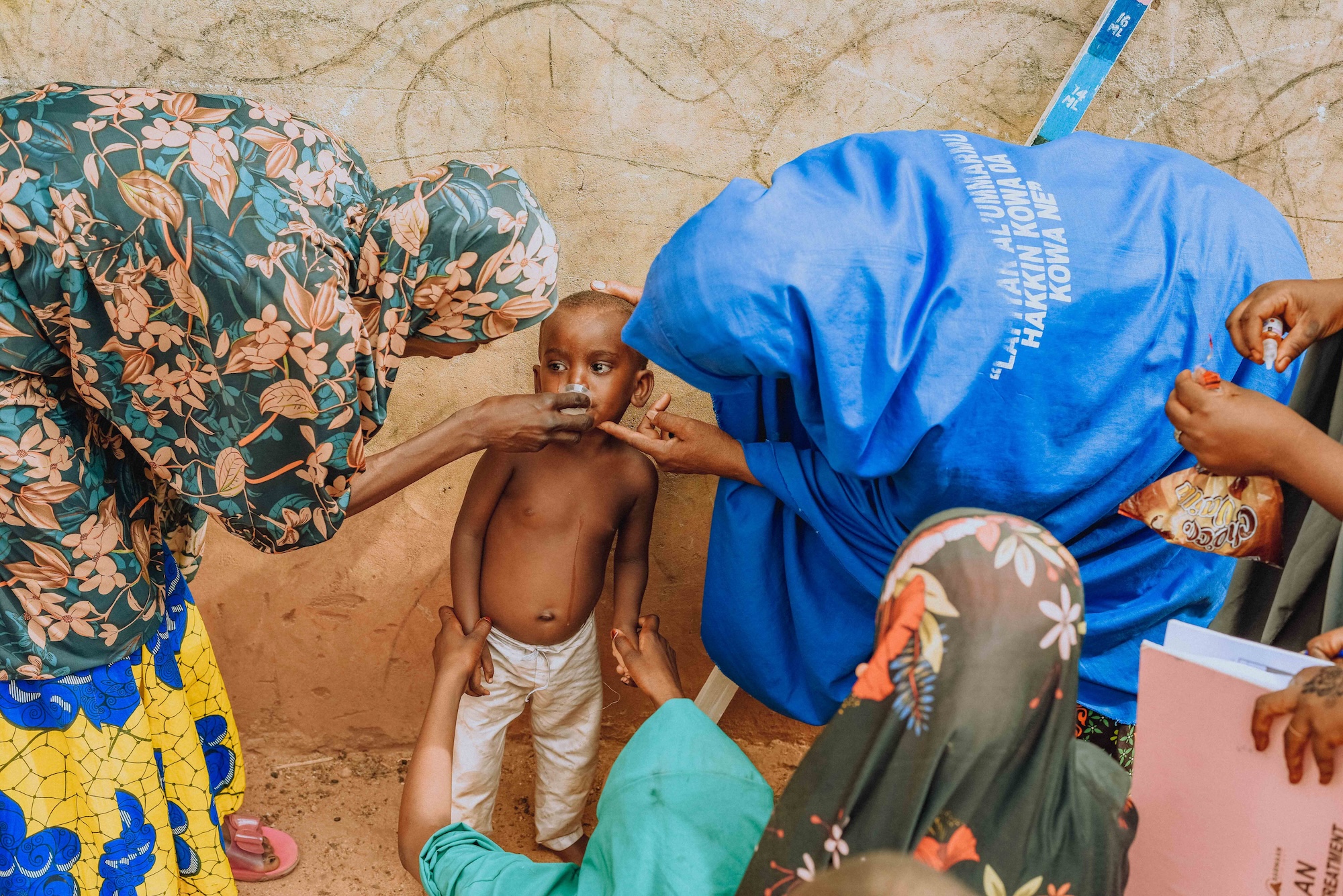
Results of azithromycin administration
The three combined rounds of data collection produced the following results:
| Round (R) | Eligible children targeted | Children treated | Coverage rate |
| R1 | 482508 | 453006 | 93.9% |
| R2 | 490577 | 466827 | 95.2% |
| R3 | 489804 | 469031 | 95.8% |
Lessons learned
- Sensitizing traditional and religious leaders fostered community dialogue and participation which greatly improved acceptance of azithromycin in the community.
- Azithromycin MDA is safe, as no serious adverse events were reported in children who received the intervention.
- Sustainability is promising, as a significant proportion of participating parents and guardians expressed willingness to pay for the drug even after the conclusion of the intervention.
SARMAAN II
In its drive to consolidate efforts to reduce under-5 mortality rates (U5MR), Nigeria will be scaling up azithromycin MDA in seven high U5MR burden states with infant mortality rates >60 per 1000 and/or U5MR >80 per 1000 live births.
To this end, the country will ensure rigorous assessment of the intervention on child survival, continuous monitoring
of antimicrobial resistance and identify measures towards sustainability. This is borne out of evidence emerging from the SARMAAN I pilot and AVENIR studies which reveals a favourable safety profile and a positive impact on mortality.
SARMAAN II will use a full-powered type III hybrid effectiveness implementation study design and the RE-AIM framework (Reach, Effectiveness, Adoption, Implementation, Maintenance to assess the effectiveness of azithromycin MDA in the reduction of U5MR and its impact on antimicrobial resistance.
The Nigerian Institute of Medical Research is leading the research work in collaboration with implementing partners, state governments (Kebbi, Sokoto, Kano, Kebbi, Katsina, Kaduna, Jigawa and Bauchi) and allied agencies of the Federal Ministry of Health for prompt translation of findings into policy.

SARMAAN II:
Objectives
- Evaluate the coverage and equity of mass administration of azithromycin (Reach)
- Evaluate the child health outcome (mortality and morbidity) among children aged 1–59 months over the study period
- Determine the antimicrobial resistance and safety of azithromycin MDA in children aged 1–59 months (Effectiveness)
- Determine factors influencing implementer readiness to adopt azithromycin MDA as a policy (Adoption)
- Explore and understand the implementation processes, challenges, and successes of azithromycin MDA when co-administered with other child survival strategies across all project sites (Implementation)
- Establish best practices for monitoring the safety and antimicrobial resistance of azithromycin MDA at scale within routine settings (Maintenance).
SARMAAN II:
Study design
Two types of study design will be adopted to ensure adequate reach of all children and robust evaluation of the impact of intervention on U5MR. Furthermore, safety coverage, AMR, morbidity, fidelity to the implementation, and sustainability plans will be evaluated.
- Community-based prospective study:
The community-based prospective study design will entail a six-monthly implementation of MDA intervention for all eligible children aged 1–59 months across all local government areas in the six states of Bauchi, Kaduna, Katsina, Kebbi, Jigawa and Sokoto. - Stepped-wedge randomized cluster design:
The project will adopt a stepped-wedge study design in Kano State. The design will ensure equity and the ethical requirement to not leave any under-5 child behind during the roll-out phase for azithromycin MDA.
Number of children targeted per cycle:
| Sokoto | 956322 |
| Kano | 2,427,325 |
| Kebbi | 858,696 |
| Katsina | 1,535,871 |
| Kaduna | 1,608,500 |
| Jigawa | 1,153,010 |
| Bauchi | 1,239,905 |
Study outcomes
Primary Outcomes
- Pattern of under-5 mortality rates
- Trends in azithromycin antimicrobial resistance patterns
Secondary Outcomes
- Pattern of under-5 morbidity
- Pattern of the safety profile of azithromycin MDA among children aged 1–59 months
- The facilitators and barriers to readiness for adopting azithromycin MDA as a child survival strategy in study sites
- Metrics relating to the degree of adherence to implementation activities (fidelity) for routine azithromycin MDA in the country.

SARMAAN partners
- Federal Ministry of Health Nigeria
- Nigerian Institute of Medical Research (NIMR)
- National Malaria Elimination Programme
- eHealth Africa
- National Primary Healthcare Agency
- Sightsavers
- University of Washington
- Corona Management Systems