AVENIR, Niger
AVENIR = Azithromycine pour la Vie des Enfants au Niger (“Azithromycin [to save] young lives in Niger”)

AVENIR I: Overview
The AVENIR I trial was designed to test the effect of age- based targeting on mortality and resistance with biannual oral azithromycin distribution.
- AVENIR provided an opportunity to demonstrate and rigorously assess large- scale implementation of this program.
AVENIR I: Trial Design and Objectives
The overall objectives of the trial were to determine the optimal age group to treat with biannual oral azithromycin distribution to reduce child mortality and to compare selection for antimicrobial resistance across different age- based strategies for azithromycin distribution. In a series of secondary outcomes and parallel studies, the trial also evaluated large-scale implementation.
The following were specific aims for the Mortality / Resistance trial:
- To compare the efficacy of biannual oral azithromycin distribution to reduce mortality in children 1–59 months old in communities distributing azithromycin to ages 1–59 months (azithro 1–59) compared to placebo.
- To compare the efficacy of biannual oral azithromycin distribution to reduce mortality in children 1–11 months old in communities distributing azithromycin to ages 1–11 months (azithro 1–11) compared to placebo.
- To compare the efficacy of biannual oral azithromycin distribution to reduce mortality in children 12–59 months old in communities distributing azithromycin to ages 1–11 months (azithro 1–11) compared to 1–59 months (azithro 1–59).
AVENIR used a large simple cluster-randomized placebo- controlled adaptive platform design to conduct multiple studies. AVENIR used a response-adaptive allocation to achieve several aims. By maintaining an allocation of at least 10% of communities to placebo, continued monitoring of efficacy was still possible. Eligible communities in Niger were randomly selected for participation in the Mortality / Resistance Trial, the Delivery Trials, or the Programmatic Trial.
AVENIR I: Results
Headline result:
Infant mortality was 17% lower when older children (1–59 months) also received azithromycin than when only infants aged 1–11 months received active treatment.
Mortality/Resistance Trial
“Azithromycin to Reduce Mortality – An Adaptive Cluster-Randomized Trial” was published in the New England Journal of Medicine in August 2024: https://www.nejm.org/doi/full/10.1056/ NEJMoa2312093
- Antimicrobial resistance outcomes expected Q1 2025
Delivery Trial I
“Comparison of door-to-door and fixed-point delivery of azithromycin distribution for
child survival in Niger: A cluster-randomized trial” was published in PLOS Global Public Health in November 2023: https://pubmed.ncbi.nlm.nih.gov/37967058/
Delivery Trial II
Primary results under review


AVENIR II: Overview
With several randomized controlled trials demonstrating that azithromycin mass drug administration (MDA) reduces childhood mortality, Niger is expanding the azithromycin MDA program nationwide to children aged 1–59 months.
To establish monitoring of mortality and antimicrobial resistance (AMR) as part of this program as well as to leverage the infrastructure to evaluate other child health interventions, AVENIR II is designed as an adaptive platform trial with monitoring and re-randomization every two years.
AVENIR II: Trial Design and Objectives
AVENIR II is a cluster-randomized adaptive platform trial designed to evaluate community health interventions in Niger. The initial focus is to monitor under-5 mortality and antimicrobial resistance as the azithromycin MDA for child survival program expands in Niger. There are two specific aims:
- Mortality
- To conduct surveillance of mortality over time compared to the Sustainable Development Goal targets for under-5 mortality reduction. As this intervention is not intended to continue indefinitely, surveillance against a target is needed to determine when to stop.
- To continue to evaluate the effectiveness of azithromycin MDA to reduce under-5 mortality. Given the risk of AMR, evaluation of the effectiveness of the intervention over time is needed to fully weigh the risks against the benefits.
- Antimicrobial Resistance
To determine the impact of azithromycin MDA on AMR in population- and clinic-based samples.
AVENIR II:
Expansion Plan and Details
The map shows the regions of Niger coloured according to the progressive scale-up plan. Borders indicate the seven regions in Niger plus the capital, Niamey, which is not included in the scale-up, as urban areas are excluded from this study. Dosso, Maradi, and Tahoua received the first round of treatment during the third quarter of 2024. Zinder will receive the first round of treatment in the fourth quarter of 2024.
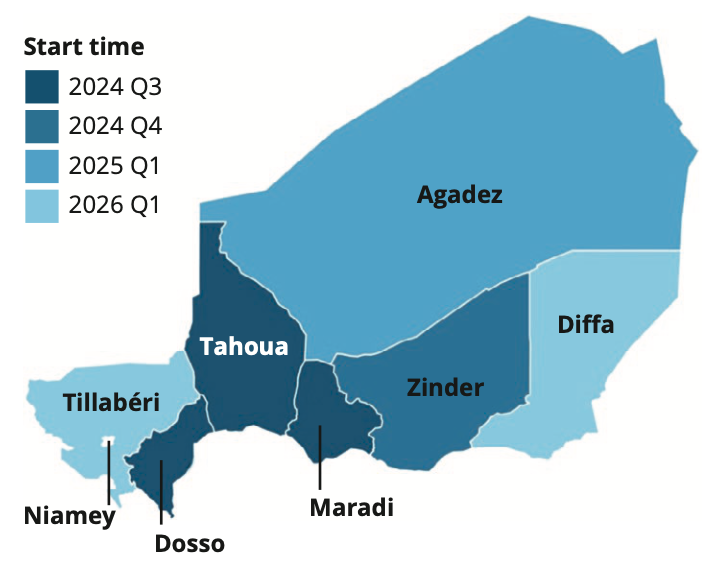
Map of Niger coloured according to the AVENIR II scale-up plan
AVENIR II:
Design and Monitoring
Primary health centre catchment areas (“Centre de Santé Intégrés”, CSI) are randomized to receive azithromycin MDA or delayed treatment in a stepped wedge design for the first two years. Rural CSIs from all seven regions will be included in the trial, which we expect to include 1,328 CSIs and approximately 3.3 million children 1–59 months. 10% of CSIs will be randomized delayed treatment to start, and an equal number of CSIs will be randomly selected for monitoring.
Mortality and AMR will be monitored at baseline and every two years. To enable continued monitoring while ensuring access to treatment, CSIs receiving MDA will be re-randomized using the adaptive algorithm updated with new mortality results and no CSI will go without treatment for more than two years.
The intervention involves biannual oral azithromycin MDA to children 1–59 months old, distributed door-to-door by community health workers. The delayed intervention arm will receive usual care for the first two years, then will receive the intervention for the next two years.

AVENIR II:
Next Steps
You can read more about the progress of AVENIR II here.

Q4 2024
- Second MDA in Dosso, Tahoua, Maradi
- First MDA in Zinder
- Test electronic MDA data collection via ScanForm
- Explore opportunities for integration

2025
- Continue MDAs and implementation monitoring
- Refine protocol and test integrated platforms.