LAKANA, Mali
A large-scale trial conducted in Mali to determine whether the mass administration of azithromycin to asymptomatic children can reduce the high mortality rate of 1–11-month-old children.

Overview
Data collection is currently scheduled to end mid-January 2025 and primary results are expected by the end of January 2025.
AMR and feasibility results are expected by September 2025.
LAKANA is a cluster-randomized, placebo-controlled, double-blind, three-arm trial (control (placebo), two-dose azithromycin, four-dose azithromycin).
It has the following principal research questions:
- Does biannual azithromycin (two-dose azithromycin) MDA to 1–11-month-old infants reduce their mortality in a Mali-like setting?
- Does quarterly (four-dose azithromycin) MDA have a bigger effect than two-dose MDA?
- Do the following modify the effect of azithromycin MDA on mortality?
- Cluster-level baseline mortality or coverage of seasonal malaria chemoprophylaxis (SMC)
- District of residence or distance from the nearest health facility
- Household asset or income index, household WASH index
- Season of MDA and time since Seasonal Malaria ChemoprophylaxisInfant sex, age or WAZ at the time of MDA
- Does two-dose or four-dose azithromycin MDA to 1–11-month-old infants reduce mortality among 12–59-month-old children?
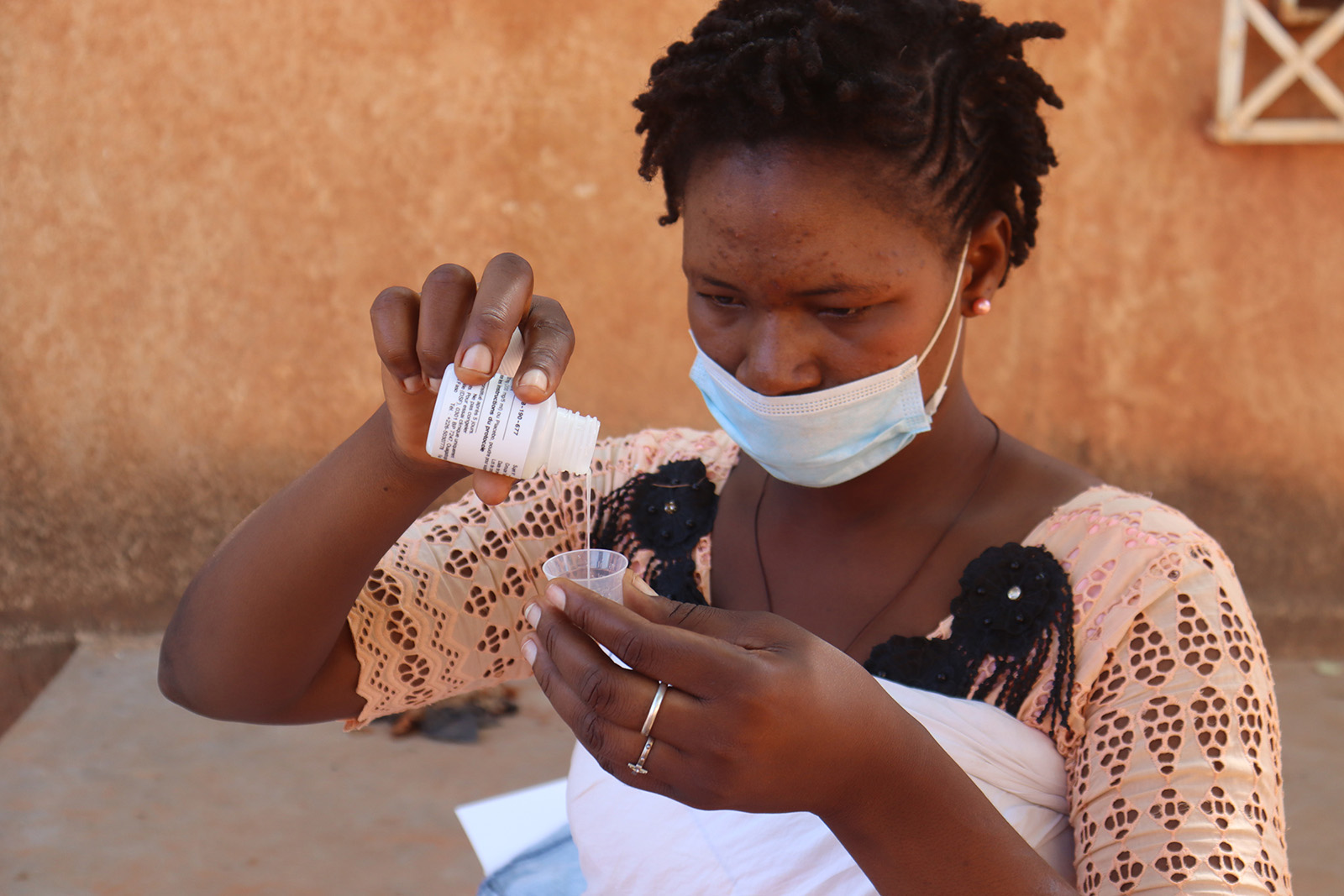
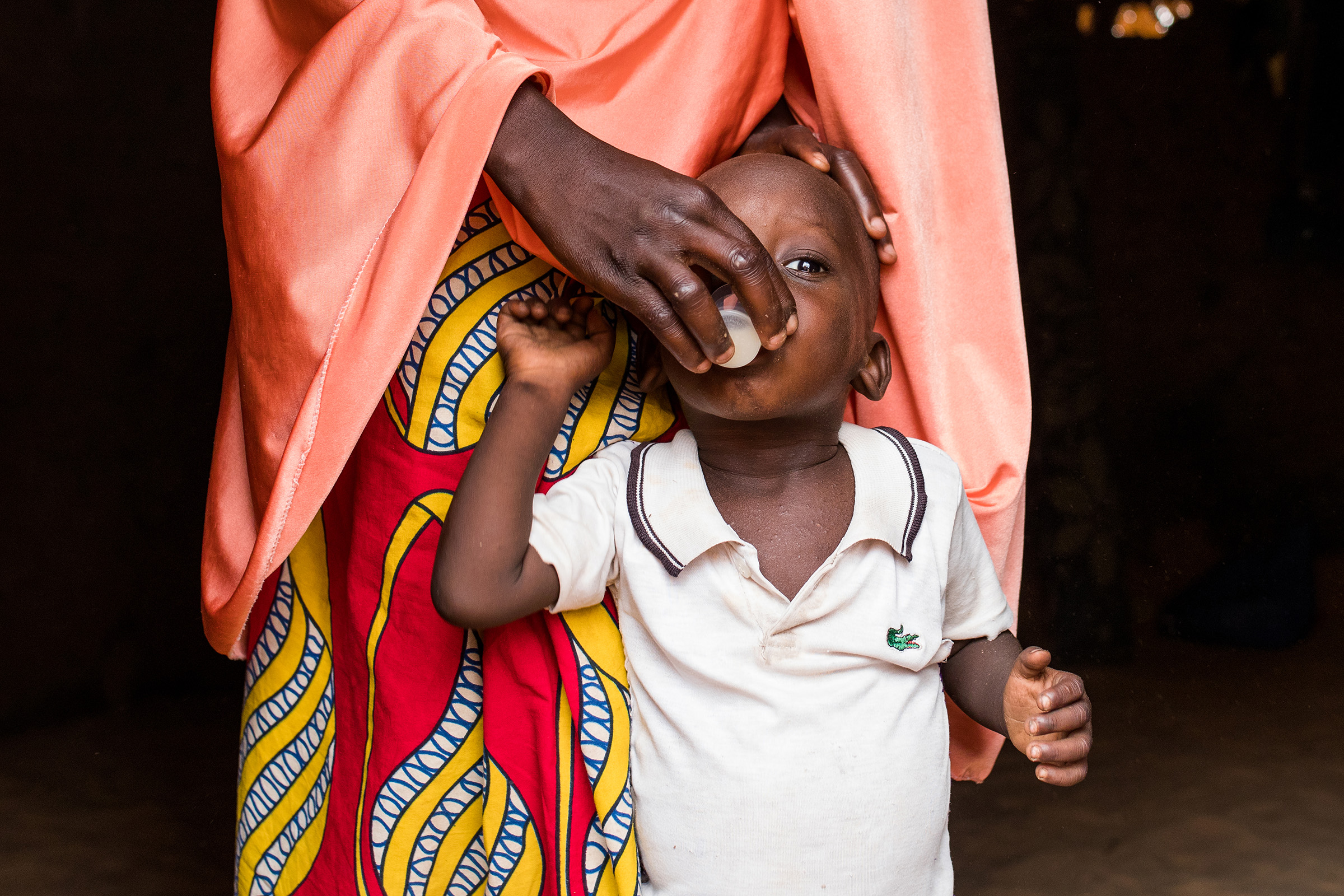
The study is conducting quarterly village visits (nine in total) for a total of two years. At every visit, census and records of births and deaths in all households are collected. The study also weighs and treats 1–11-month-old infants with 20 mg / kg of azithromycin or placebo.
1,151 villages have been followed for one or two years already (40,000 infants / 75,000 PYR).
The LAKANA study is entering its final phase, following a challenging implementation which included COVID-19 interruption and security constraints.
No serious adverse events have been recorded in the study.
Scale-up
Mali is currently in the process of scaling up azithromycin MDA implementation across the country, putting in place a national rollout to target children aged 1–59 months throughout the country (excluding the capital district of Bamako).
This implementation will be integrated with the nutrition platform, but its adaptive design will enable it to be integrated with other platforms as well. A robust monitoring and evaluation (M&E) framework will be put in place based on the lessons of other REACH countries. The M&E plan, in conjunction with national provision, will include sentinel sites, monitoring of births and deaths during MDA provision, and endline surveys. It will require providers to conduct a six-month birth and death history with each round of MDA.
Also being planned is a cross-sectional study, consisting of a small delayed-treatment population as comparators, using full pregnancy history, pre- and post-implementation. AMR monitoring will cover asymptomatic cases (rectal and nasal samples, S. pneumoniae and E. Coli) and there are plans also to isolate pathogens in order to assess pathogen-specific AMR.