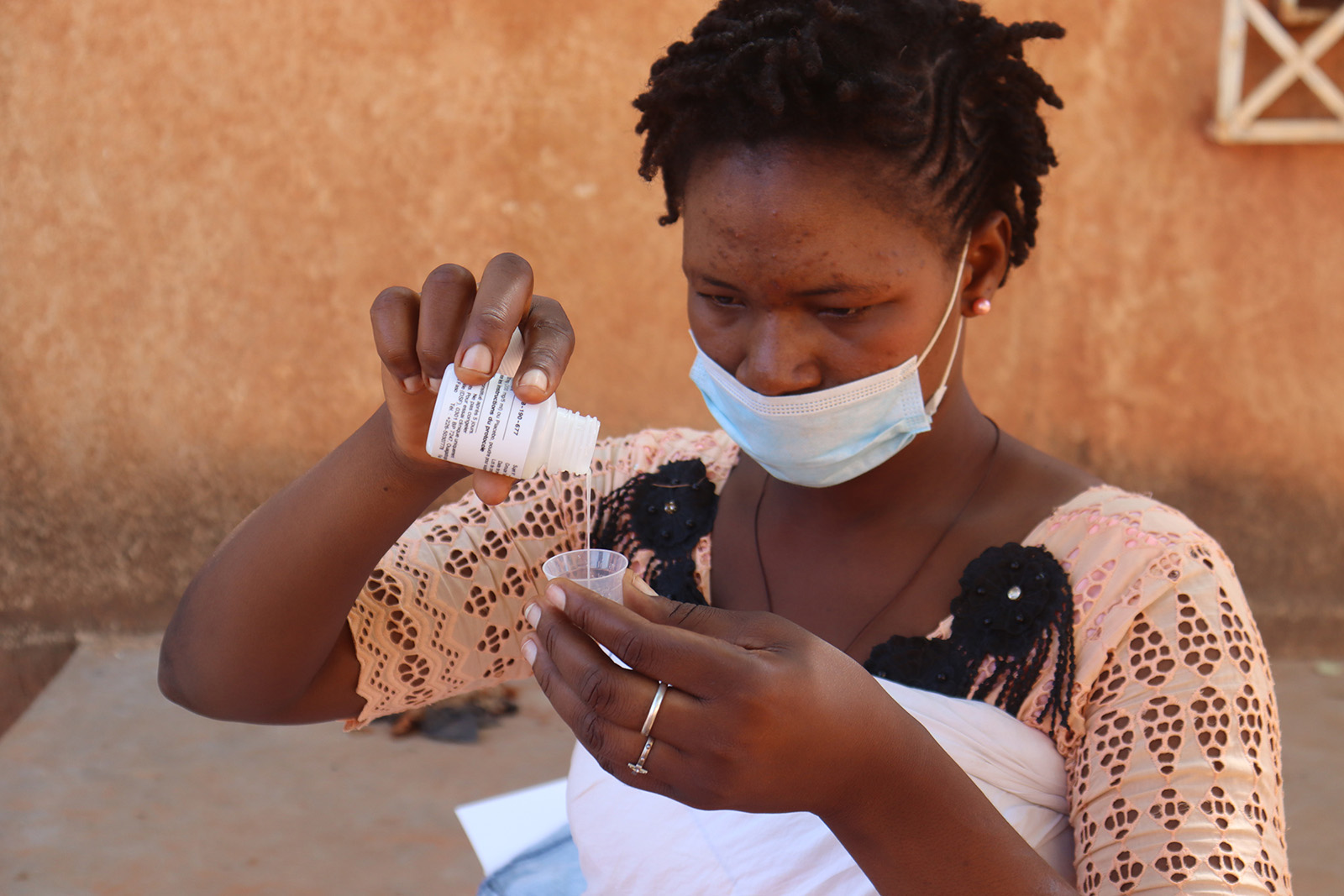
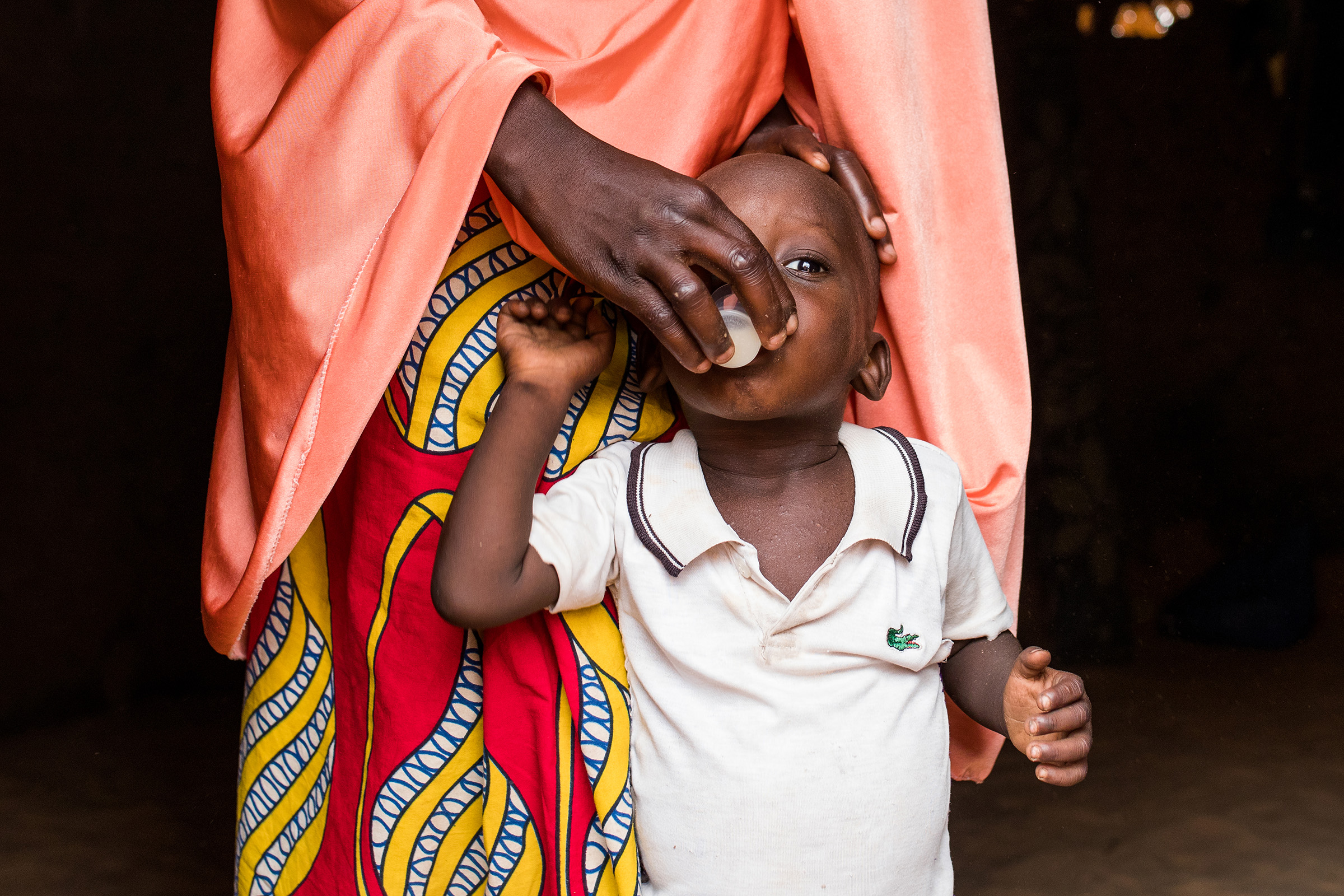
REACH Côte d’Ivoire

Background
The REACH Côte d’Ivoire project was a three-year implementation research project (2022– 2024).
Its aim was to to examine implementation of single-dose azithromycin delivered to children aged 1 to 11 months residing in high-mortality regions of Côte d’Ivoire through bi-annual mass drug administration (MDA) campaigns.
The study examined delivery integrated with existing trachoma MDAs, integration with Vitamin A delivery, and standalone MDAs.
Methods
The study followed implementation of biannual MDAs over the three-year period. Measures collected by the study were guided by the RE-AIM implementation science framework.

Reach
The intervention’s Reach was assessed through both administrative coverage estimates using available population data and periodic post-MDA coverage surveys.
Effectiveness
Effectiveness in terms of mortality was not directly examined, rather the project aimed to work closely with the Directorate of Health Information (DIS) to examine the potential of the existing health management information system, which adds community-based mortality data collection, to monitor the intervention’s primary outcome, namely infant and under-5 mortality. Additionally, under effectiveness, we assessed antimicrobial resistance (AMR) through two annual cross-sectional surveys in intervention areas, during which nasopharyngeal specimens were collected from infants who were eligible for the MDA.
These specimens were tested for the presence of resistant streptococcus pneumoniae, with a focus on resistance to macrolide antibiotics (erythromycin and azithromycin). Lastly, we monitored safety through routine MDA adverse drug event reporting mechanisms and inquired about side- effects during post-MDA coverage surveys.
Adoption
Adoption – the extent to which stakeholders are willing to adopt the intervention – was assessed qualitatively.
Implementation & Maintenance
We assessed Implementation through measures of fidelity to design. Finally, for Maintenance, we assessed acceptability from both service delivery and demand perspectives, and estimated costs per dose delivered, in order to inform future implementation.
Key Results
Reach
Six rounds of MDA were carried out, including one integrated trachoma/REACH MDA and three standalone MDAs covering 19 health districts. Additionally, the project carried out one pilot integrated Vitamin A/REACH MDA in seven health districts (four of which overlapped with the previous MDAs), followed by a full-scale Vitamin A/REACH integrated MDA in 16 health districts.
In total, the intervention delivered approximately 750,000 doses of azithromycin over six rounds of MDA in 24 health districts. Administrative estimates of coverage ranged from 48% (integrated trachoma/REACH MDA) to greater than 100% for all remaining MDAs.
Estimated coverage of the target populations was considerably lower based on post-MDA coverage assessments, ranging from 59% to a high of 89% (following the pilot Vitamin A/REACH integrated MDA).
Effectiveness
The Directorate of Health Information carried out a workshop to develop validation
rules for community indicators for the DHIS2, including mortality data, supportive supervisions in 17 health districts to monitor data collection, and data quality assessments (DQA) in 17 health districts. Findings from the DQAs were that community-based mortality data collection is making progress, but challenges exist, including high turnover for CHWs; CHWs facing heavy workload, a lack of equipment and supplies, and low remuneration; potential duplicate recording between community and facility reporting of deaths; and data validation of community data at health facility not always happening as intended.
Two rounds of AMR surveillance on prevalence/carriage of S. pneumoniae in July 2022 (three months post MDA) showed figures of 29% and 31% in November 2023. Azithromycin resistance (with erythromycin as a proxy) amongst those positive for S. pneumonia was at 34% in the first survey and 26% in the last survey.
Adoption
Qualitative research with parents/caregivers and community-based intervention personnel (community drug distributors and their supervisors) and district personnel involved in the MDAs, as well as with national- level stakeholders, indicated that the intervention was highly acceptable. Both district and national stakeholders expressed a need for accurate mortality monitoring data to understand the population impact of the intervention should it be implemented routinely at scale.
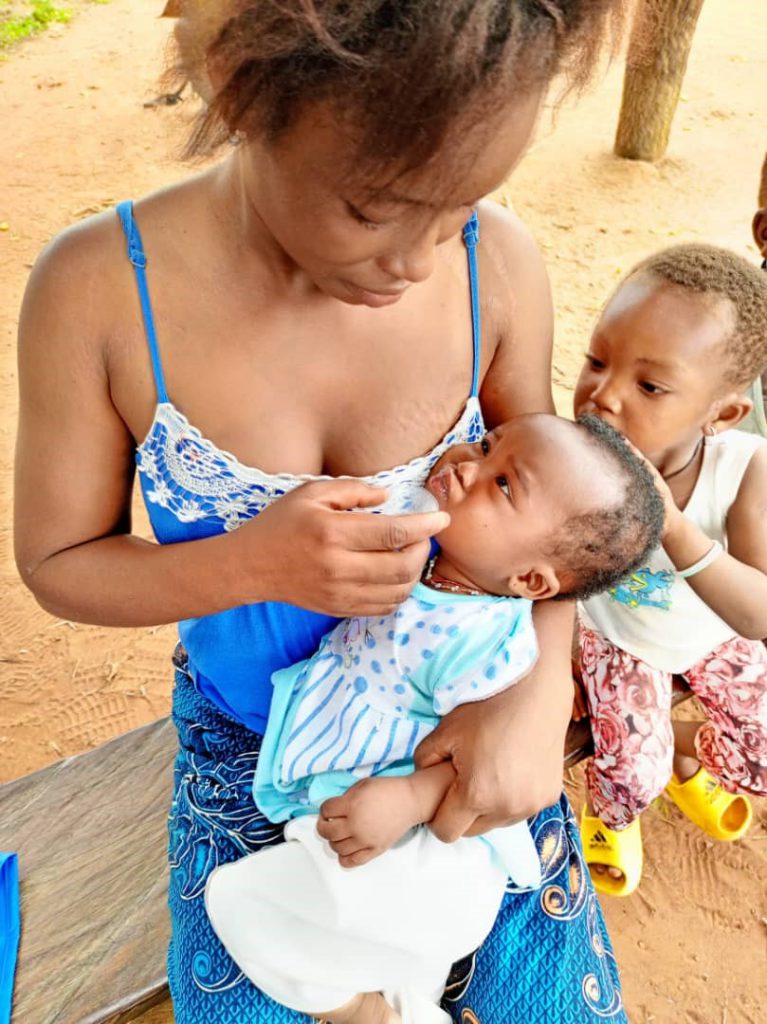
Implementation
MDAs were carried out largely according to design in terms of activities at the community level. Key challenges to implementation included:
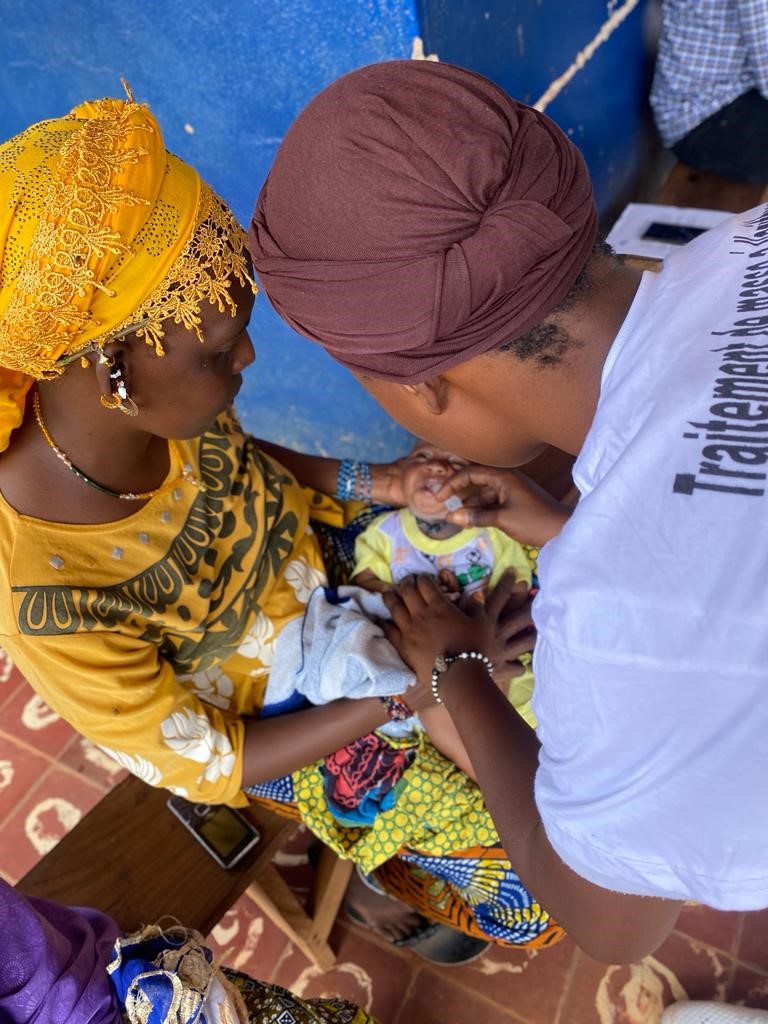
Competing priorities with national programs, which led to delays in delivery. Although six rounds of MDA were carried out, they did not take place at regular intervals.
Although the intervention ultimately reached 24 health districts, this was fewer than half of those that would qualify for the intervention per 2020 WHO guidance. Remaining districts would need to be reached either through other platforms or by standalone MDAs.
Integrated delivery provided minimal cost-savings.
Monitoring coverage through administrative data proved inaccurate.
Maintenance
Cost analysis of different delivery strategies revealed that the financial cost for delivery of one dose of azithromycin ranged from $2–$2.50 per child. These costs do not include the non-financial costs that would be required to implement the programme.

Summary
- Azithromycin MDA to reduce child mortality is a highly acceptable and feasible intervention to implement.
- Integrated delivery with another mass distribution campaign to deliver azithromycin, while intuitively more efficient, is not always so in practice. The main factors that drive synergies in integrated delivery include alignment between the two programmes in terms of geographic distribution of the target populations, the actual populations being targeted, and the interventions themselves.
- Cost-efficient mechanisms to monitor both mortality and AMR will be required for the country to take up the intervention.
Next steps
If the intervention is to be scaled up, it will be important to decide on the overall target population, both in terms of the ages of the children to be targeted (given newer research findings) and the target geographic areas.
A formal impact evaluation where implementation and its outcomes (mortality and AMR) are strictly monitored will be critical to understand if the effects seen in clinical trials translate into reductions in mortality when the intervention is delivered under real world conditions.
Simultaneous strengthening of health information systems that permit routine monitoring of both AMR and mortality are essential to long-term implementation. Testing possible options for both is recommended.
REACH Côte d’Ivoire Study Partners
- FHI 360
- National Programme for the Control of Neglected Tropical Diseases with Preventive Chemotherapy
- Health Information and Information Technology Department
- Pasteur Institute of Côte d’Ivoire National Nutrition Programme