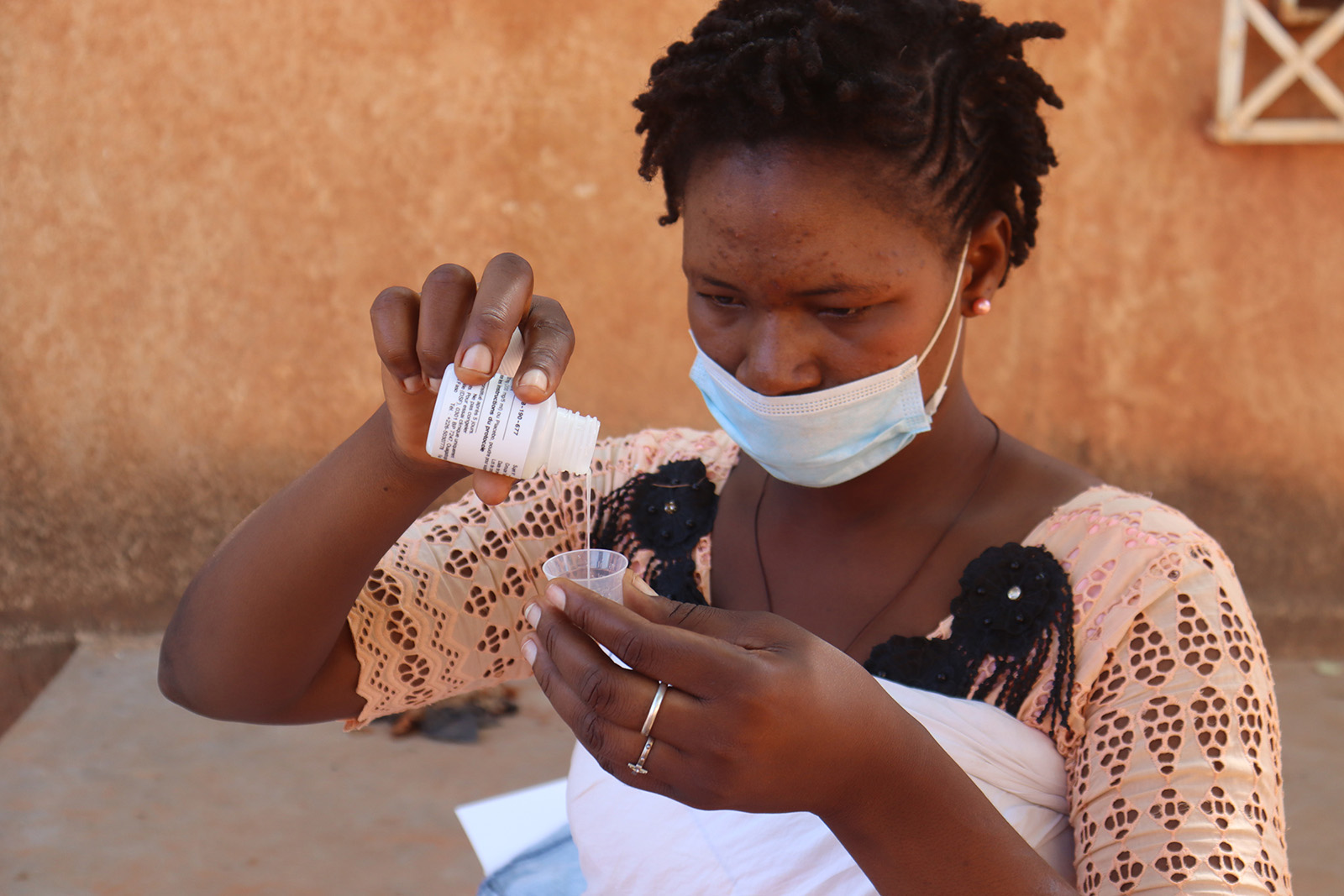
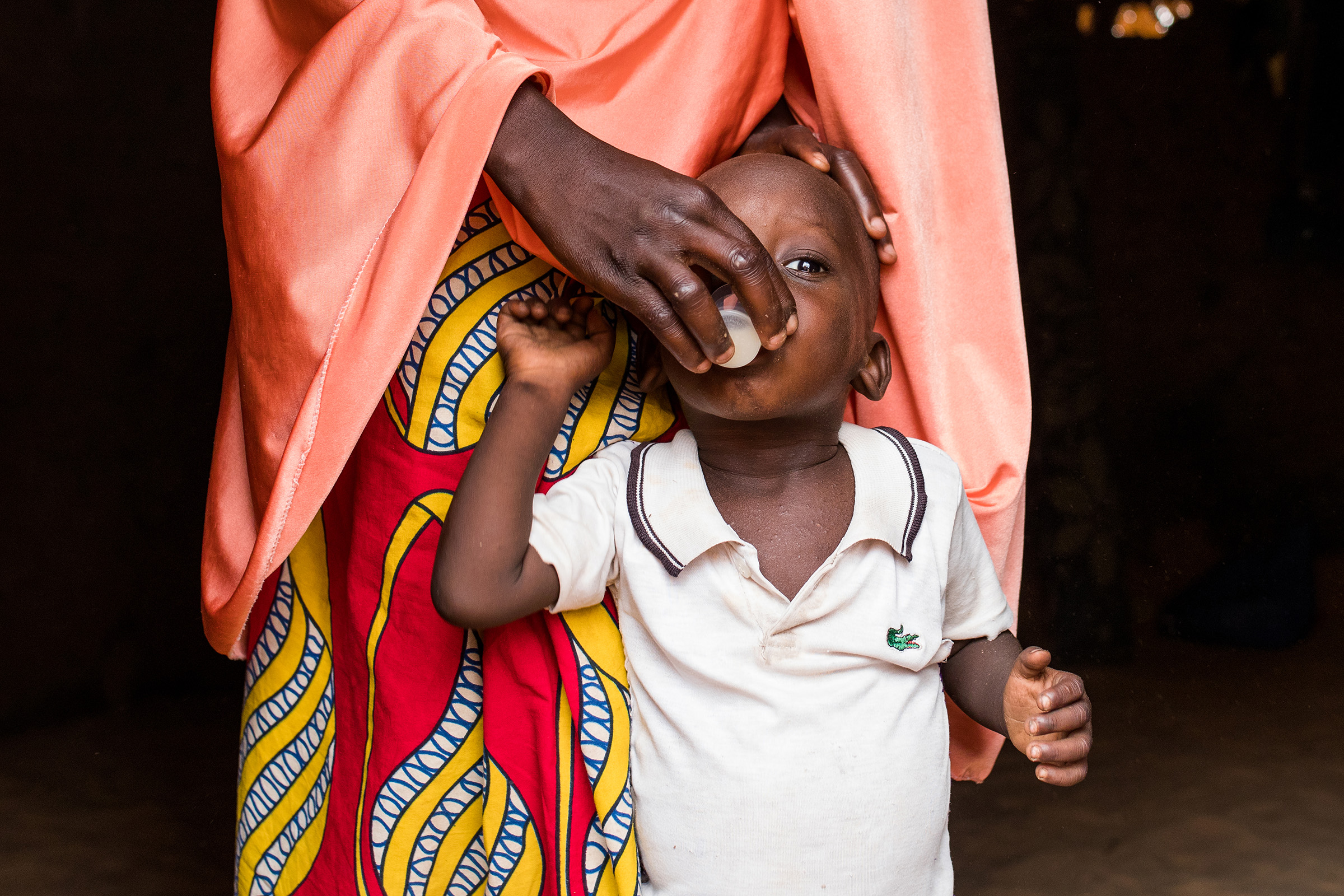
MIRAMA, Burkina Faso
MIRAMA = Mortalité Infantile Reduite par l’Administration de Masse de l’Azithromycine (“Reducing Infant Mortality through Mass Administration of Azithromycin”)

Background
Despite progress in recent decades, infant mortality remains high in Burkina Faso. Down from 85‰ in 2006 to 49.41‰ in 2021, this remains one of the highest child mortality rates in the world.
To reach the target of 25 under five deaths per 1,000 live births, it has become a matter of great urgency to put in place effective interventions and to improve health systems.
This project is being implemented from June 2021 to March 2025 by the Ministry of Health through its Family Health Directorate, in collaboration with Helen Keller International and with funding from the Gates Foundation.
Following the results of the MORDOR trial, Burkina Faso has adopted the mass administration of azithromycin to children aged 1 to 11 months, integrated with the Vitamin A+ platform (JVA+), in the form of a randomised, double-blind, placebo-controlled trial.
Overall objective
MIRAMA’s overall objective is to assess the impact of twice- yearly azithromycin on infant mortality when integrated with the Vitamin A+ intervention platform in Burkina Faso.
Specific objective 1
To evaluate whether azithromycin integrated with the Vitamin A+ platform reduces mortality in children aged between 1 and 11 months, compared with placebo.
Specific objective 2
- To compare the cluster-level prevalence of macrolide- resistant pneumococci in nasopharyngeal samples taken from children aged 1–59 months in clusters receiving azithromycin versus clusters receiving placebo.
- To compare the burden of genetic determinants of macrolide resistance in rectal samples taken from children aged 1–59 months in groups receiving azithromycin versus groups receiving placebo.
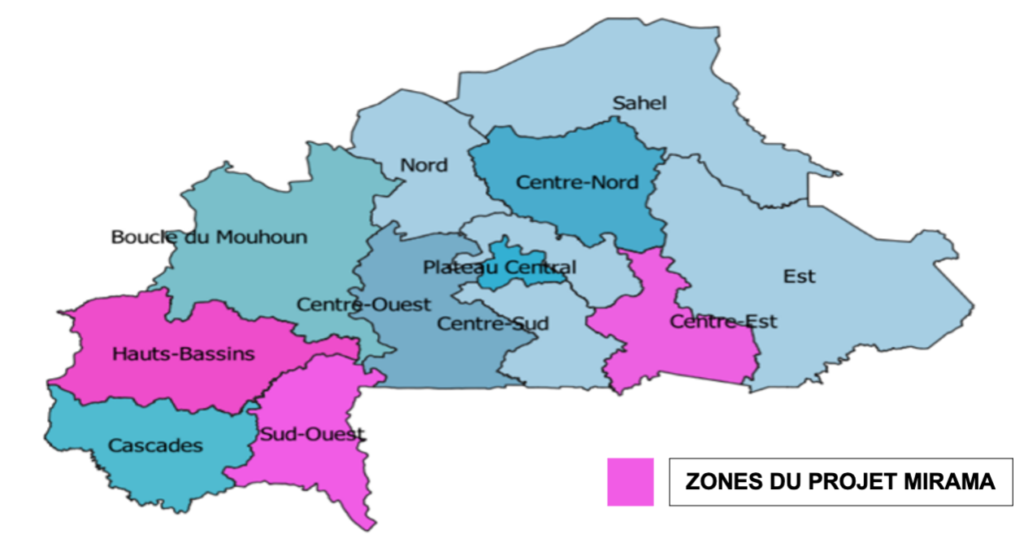
Methodology & Study design
Study area
The study’s intervention areas are the Hauts Bassins, Sud- Ouest and Centre-Est regions of Burkina Faso.
Mortality
This is a cluster-randomised, double-blind, placebo- controlled trial with a 1:2 split in favour of active treatment. All communities in the target area are selected to participate in the mortality study.
Resistance
60 health facilities (CSPS) were randomly selected
from the study area to receive resistance monitoring, maintaining a 1:2 allocation ratio. In each CSPS, one community was randomly selected for sample collection. Antimicrobial resistance monitoring took place before the first distribution and will take place again after the last distribution.
Sample size
20 districts, around 2,595 villages, with some 105,000 children aged between 1 and 11 months.
Resistance sample size
40 azithromycin communities and 20 placebo communities (2:1 split).
Verbal autopsy
Starting with the 3rd census round, a verbal autopsy was carried out on all children who died during the previous census periods, using WHO’s 2016 Verbal Autopsy questionnaire. The verbal autopsy interviews were conducted by staff trained by the project team and the Nouna Health Research Centre (CRSN).

Statistical analysis
Mortality
Mortality is analysed using Poisson regression and standard methods for cluster randomised trials.
Antimicrobial Resistance
The results measured are:
- Prevalence of macrolide-resistant pneumococcal isolates from nasopharyngeal samples in children aged 1–59 months, at baseline and at 24 months.
- Loads of the genetic determinants of macrolide resistance, including determinants known to be present in Campylobacter spp, Salmonella spp, Shigella spp and Escherichia coli, from rectal samples taken from children aged 1–59 months, at baseline and at 24-months.
Cost-effectiveness study
A survey was carried out to determine the cost of combining the prophylactic administration of azithromycin with the Vitamin A+ platform. The study report is currently being finalised.
Health-system strengthening
Capacity-building for the various participants/actors was carried out at all levels (central, intermediate, peripheral). The project coordination team supervised the various training courses to ensure their effectiveness.
A number of the Ministry of Health’s structures have benefited from strengthening, both in terms of infrastructure and equipment.
Monitoring and surveillance committees
Two committees have been set up:
- A technical monitoring committee, which meets quarterly to monitor the implementation of the project and to ensure effective coordination and collaboration with research institutes and centres.
- A Data and Safety Monitoring Board (DSMB) which has met once a year to review the study’s progress.
Main achievements to date
- 4 mass administration campaigns of azithromycin integrated with the Vitamin A+ platform
- 5 rounds of census and vital status monitoring, the last of which is intercut with the birth history survey
- 3 surveys of morbidity data in health centres
- 3 verbal autopsy investigations
- 2 sample collection surveys to monitor antibiotic resistance.
Study timetable
All study results will be available during the first quarter of 2025.
MIRAMA project stakeholders
- Ministry of Health
- University of California, San Francisco (UCSF)
- Nouna Health Research Centre (CRSN)
Scale-up
The scale-up of REACH activities has been endorsed by all stakeholders and relevant authorities.
This plan for scaling up the prophylactic mass administration of azithromycin to children aged between 1 and 11 months in Burkina Faso, scheduled for the period 2024–2029, aims to gradually cover all 70 districts in the country’s 13 regions over a period of six years, and will be fully integrated with the Vitamin A+ platform.

MIRAMA in the field
Training of Community Distributors on the implementation guidelines for administering azithromycin before a combined JAV+ / Azithromycin campaign, Burkina Faso, South-West Region, June 2022

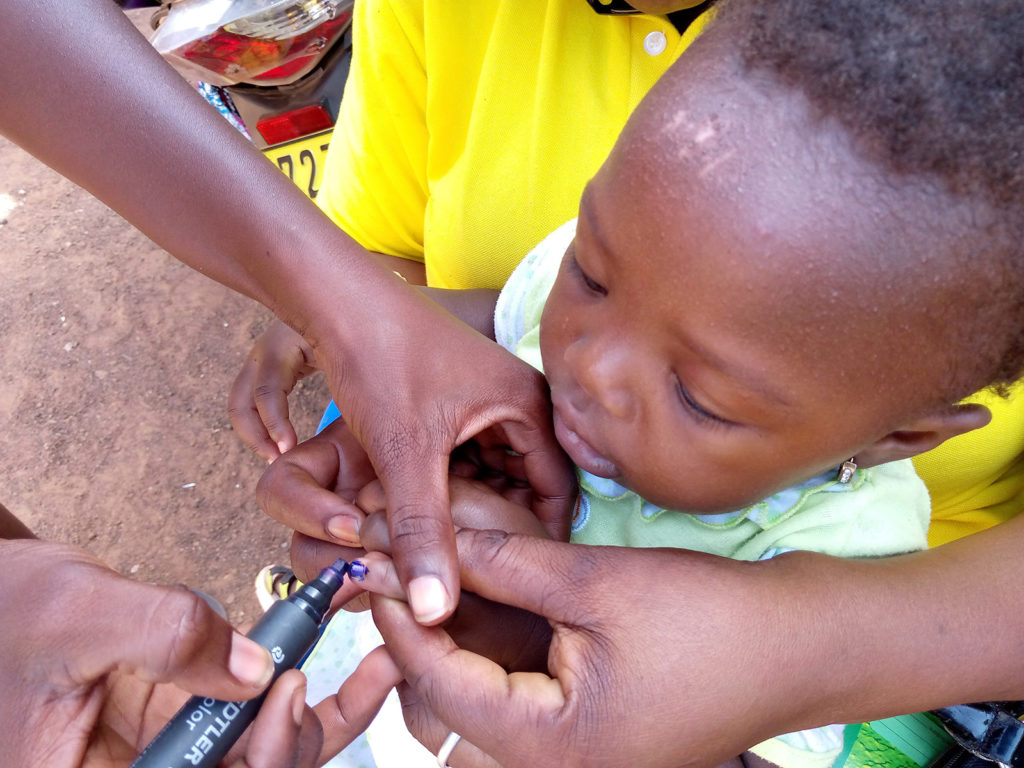
Marking a child’s finger with indelible ink after monitoring vital status, South-West region, Kampti, October 2022