Sierra Leone, ICARIA
Improving Care through Azithromycin Research for Infants in Africa
An efficacy trial of the incremental mortality benefit of IPTi plus azithromycin
Background and justification
Infectious diseases are among the most common causes of mortality in the over 2.5 million children under 5 years of age (U5) who died in 2018 in sub-Saharan Africa. New approaches to treatment and prevention of these diseases are needed to increase child survival. Sierra Leone has one of the highest rates of U5 child mortality in the world. It is estimated that 32,000 children die each year, the leading causes of death being neonatal conditions, malaria, pneumonia and diarrhea. In Sierra Leone, available information on the malaria burden indicates that this accounts for 38% of deaths among U5 children.
Recent studies have shown that azithromycin – a macrolide antibiotic with some antimalarial effect- is associated with significant reductions in childhood mortality when used in mass drug administration (MDA) for trachoma elimination in some sub-Saharan African countries with child mortality rates far beyond Sustainable Development Goal targets.
However, despite the potential benefit of the intervention, several fundamental scientific questions remain to be answered before it can be recommended for large-scale implementation.
Intermittent Preventive Treatment for malaria in infants (IPTi) with sulphadoxine-pyrimethamine (SP), now renamed by the World Health Organisation (WHO) as Perennial Malaria Chemoprevention (PMC) has been shown to reduce clinical malaria in infants. IPTi-SP constitutes one of the most cost-effective strategies for malaria control. In 2010, WHO recommended IPTi for malarial control in children living in areas with moderate-to-high transmission where resistance to SP is not high (defined as ≤50% prevalence of the Plasmodium falciparum enzyme dihydropteroate synthase (dhps) gene K540E mutation) and being co-administered with DTP2, DTP3 and measles immunization. Until recently, Sierra Leone was the first and only country that had implemented the IPTi-SP since 2018 nationwide.
Expansion of malaria protection into the second year of life through IPTi may be beneficial in line with the updated WHO recommendations for malaria chemoprevention among children.
In order to increase cost-effective strategies that improve child survival in settings with the highest mortality burden, this trial aims to assess the impact of azithromycin administered alongside IPTi-SP through the Expanded Programme on Immunization (EPI) scheme on overall mortality up to 18 months of age in Sierra Leone.
Objectives
General objective
To evaluate the impact of azithromycin administered through the Expanded Program of Immunization in addition to IPTi-SP on all-cause mortality at 18 months of age in Sierra Leone.
Specific objectives
1. To evaluate the effect on all-cause mortality up to the age of 18 months of adding azithromycin at 6 weeks, 9 and 15 months of age to IPTi-SP administered at 10, 14 weeks, 6, 9, 12 and 15 months of age alongside routine EPI immunizations
2. To assess adverse events associated with azithromycin administration
3. To assess the potential development of resistance to macrolides
4. To determine the potential interactions between azithromycin and specific routine EPI immunizations (Measles, Rubella and Yellow Fever)
5. To determine the effect of the interventions on malaria-related indicators
6. To assess the potential risk of development of SP parasite resistance
7. To examine the health system implications related to the addition of azithromycin to the EPI and its acceptability by health providers and participants.
Methods
Study design
The study is a 2-arm individually randomized double blind placebo-controlled clinical trial of azithromycin administration in young children in Sierra Leone.
Study arms:
1. Azithromycin at DTP-1 visit at 6 weeks of age, azithromycin (plus IPTi)* at measles visit at 9 months of age and azithromycin (plus IPTi)* at measles booster visit at 15 months of age.
2. Placebo at DTP-1 visit at 6 weeks of age, placebo (plus IPTi)* at measles visit at 9 months of age and placebo (plus IPTi)* at measles booster visit at 15 months of age.
*IPTi will be administered in both study arms at 10 and 14 weeks of age and at 6, 9, 12 and 15 months of age.
Study endpoints
Primary endpoint
All-cause mortality rate at 18 months of age
Secondary endpoints
1. Cause-specific mortality rate at 18 months of age
2. Malaria-related mortality at 18 months of age
3. Incidence of all-cause hospital admissions
4. Incidence of all-cause outpatient attendances
5. Incidence of confirmed (RDT positive) malaria outpatient attendances
6. Incidence of confirmed (blood smear positive/RDT positive) malaria hospital admissions
7. Frequency and severity of drug adverse reactions
8. Prevalence of SP resistance in U5 children
9. Prevalence of macrolide resistance in nasopharyngeal isolates
10. Prevalence of macrolide resistance in the gut bacteria
11. Proportion of children with protective antibody responses to specific routine EPI immunizations (measles, rubella and yellow fever)
Study location
The trial will be conducted in 3 districts of Sierra Leone, namely Bombali, Port Loko and Tonkolili, and will involve 14 primary health care facilities across these districts.
Sample size
A sample size of 20,560 infants (10,280 per group AZi / placebo) has been estimated to achieve a 16.6% reduction in all-cause mortality at 18 months of age, with 80% statistical power and alpha error of 0.05.
Study population
The study population will be infants attending EPI services for Penta 1 vaccine administration at 6 weeks of age.
Inclusion criteria
• Parents/guardians agree to participate and have signed the informed consent
• Body weight equal or above 2.5kg
• 5 to 10 weeks of age at the time of recruitment
• Permanent residence in the study area-health facility catchment area
• Without known allergies to or contraindications to macrolides
• Without known allergies to or contraindications to SP
• Agreement to complete the EPI scheme at the recruitment health facility
Exclusion criteria
• Participant’s weight below 2.5kg
• Aged above 10 weeks of age at the time of recruitment
• Residence outside the study area or planning to move out in the following 12 months from enrolment
• Known history of allergy or contraindications to macrolides
• Known history of allergy or contraindications to SP
• With signs of any acute illness at the time of recruitment or having been diagnosed with chronic hepatic, cardiac or renal diseases
• Participating in other intervention studies
ICARIA in the field
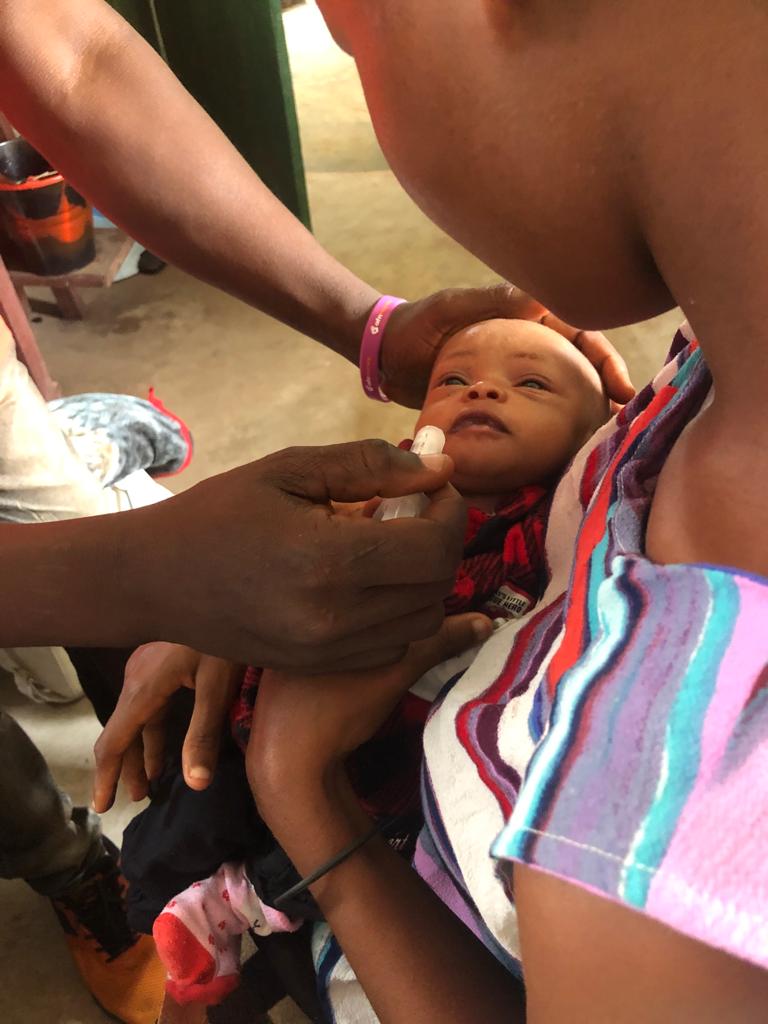
ICARIA is evaluating the impact of azithromycin administered through the Expanded Program of Immunization on all-cause mortality at 18 months of age in Sierra Leone.